FDA revokes EUA for single-dose Johnson & Johnson COVID-19 vaccine as last batch expires – but only after the Big Pharma company’s request for withdrawal
06/07/2023 / By Laura Harris
The Food and Drug Administration (FDA) has pulled the emergency use authorization (EUA) it issued for Johnson & Johnson’s Wuhan coronavirus (COVID-19) vaccine, ending its short-lived but chaotic existence during the pandemic.
The regulator announced the revocation on June 1 in the form of a letter it sent to Janssen Biotech. Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said the letter was in response to the company’s May 22 request for withdrawal. Janssen Biotech is a subsidiary of the New Jersey-based J&J.
“Janssen Biotech, Inc. has informed the FDA that the last lots of the Janssen COVID-19 vaccine purchased by the U.S. government have expired; that there is no demand for new lots of the Janssen COVID-19 vaccine in the U.S.; and that Janssen Biotech Inc. does not intend to update the strain composition of this vaccine to address emerging variants,” the letter stated.
“FDA has determined that it is appropriate to protect the public health or safety to revoke this authorization. As of the date of this letter, the Janssen COVID-19 vaccine is no longer authorized for emergency use.”
Meanwhile, the Centers for Disease Control and Prevention (CDC) had previously announced that the J&J vaccine was no longer available in the U.S. as the remaining government stocks expired on May 7. According to the public health agency, approximately 31.5 million doses of the single-dose vaccine were distributed to the states at the height of the pandemic. However, only around 19 million doses were injected – leaving roughly 12.5 million unused doses to expire.
The withdrawal of the J&J vaccine’s emergency approval aligns with the FDA’s plans to evaluate new COVID-19 vaccine formulations for the upcoming fall season. But Janssen reportedly did not request the withdrawal until May 22 – two weeks after the CDC revealed that the last government stocks of its single-dose vaccine expired.
J&J vaccine linked to serious adverse effects
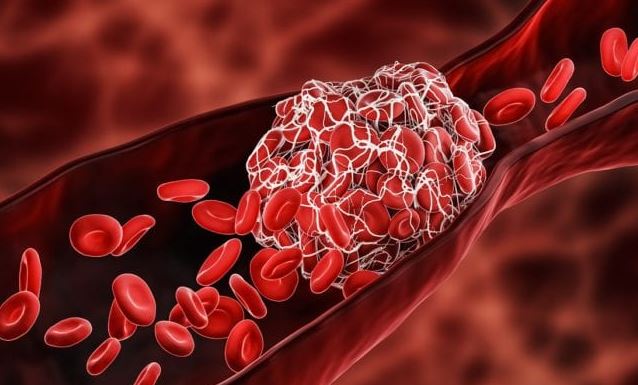
Back in 2021, the FDA and CDC paused the use of the J&J vaccine just a few weeks after it was granted an EUA. The suspension stemmed from a clotting disorder linked to the vaccine, believed to be caused by the adenovirus used to carry the SARS-CoV-2 spike protein.
The adenovirus in the single-dose shot, which the AstraZeneca COVID-19 vaccine also utilizes, triggers an immune response affecting platelets – components in the blood that are responsible for clotting. This subsequently leads to the formation of blood clots and reduction of overall platelet count. (Related: More people develop blood clots after receiving the Janssen COVID-19 vaccine.)
Aside from the blood issues, the J&J vaccine was linked to Guillain-Barre syndrome (GBS). The syndrome involves the immune system attacking the nerves, which can lead to different neurological issues and even death. The serious reaction had led the FDA to place warning labels on the vaccine, highlighting that it could increase the risk of developing GBS within the first 42 days after injection.
The pause on the use of J&J’s vaccine only lasted for 11 days, but demand for the single-dose shot never recovered after the downfall. Issues with quality control and contamination also contributed to the low uptake of the J&J vaccine.
Head over to Vaccines.news to read more stories about the J&J COVID-19 vaccine.
Watch Dr. Pam Popper explain why the FDA approves everything, including COVID-19 vaccines, without proper consideration.
This video is from the Wellness Forum Health channel on Brighteon.com.
More related stories:
FDA finally admits that covid vaccines cause blood clots.
Sources include:
Submit a correction >>
Tagged Under:
big government, Big Pharma, Blood clots, CDC, covid-19, EUA, FDA, Guillain-Barré syndrome, Johnson & Johnson, pandemic, pharmaceutical fraud, vaccine, vaccine damage, vaccine injury, vaccine wars, Wuhan coronavirus
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 VACCINE INJURY NEWS COM