Big Pharma now recruiting innocent children as guinea pigs for coronavirus vaccine experiments
10/22/2020 / By Ethan Huff
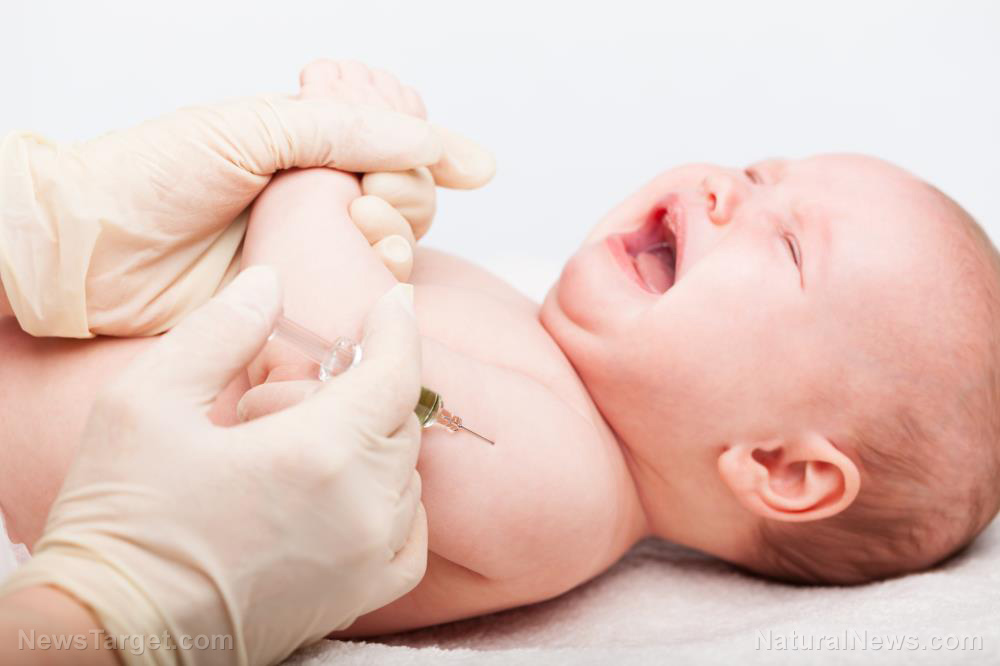
As the pharmaceutical companies participating in Operation Warp Speed round the corner towards the soon release of their vaccines for the Wuhan coronavirus (COVID-19), the push is on to start testing the jabs on the remaining and final batch of human guinea pigs: young children.
Just last week, Pfizer Inc. was granted permission to begin tests of its vaccine in American children as young as 12, joining a small handful of other companies around the world that seek to do the same. And the first young person to receive it was 16-year-old Katelyn Evans, who agreed to get jabbed at Cincinnati Children’s Hospital.
“I just figured the more people they have to do tests on, the quicker they can put out a vaccine and people can be safe and healthy,” Evans is quoted as saying.
Despite an extensive history of criminality, Pfizer earned the trust of Evans and soon many other young people who will line up to be vaccinated in order to increase the chances that a COVID-19 vaccine will be ready for children before the next school year.
While a number of other pharma giants are preparing to launch their vaccine varieties, many of these jabs are unlikely to be recommended for children, at least not at first, because more testing is needed to verify their safety and effectiveness.
“The public doesn’t understand that,” says Dr. Evan Anderson from Emory University, who for months has been pushing to test COVID-19 vaccines on children.
Because the process has apparently been moving slowly, Dr. Anderson is “very concern[ed]” that children under the age of 12 may not have a COVID-19 vaccine made available to them until next fall.
The latest news pertaining to COVID-19 and President Trump’s Operation Warp Speed program can be found at Pandemic.news.
Children have almost zero risk of COVID-19, so why do they need a vaccine?
Even though children are the least likely demographic to receive a positive COVID-19 test, Big Pharma wants them to be vaccinated just like everybody else.
Consequently, the race is on to see how many pharmaceutical companies can complete their vaccine experiments on children in enough time to get the jabs approved for the next school term.
In China, pharmaceutical companies Sinovac and SinoPharm have both launched studies to test their respective vaccines on children as young as three. A British study involving AstraZeneca’s jab has also been approved to test a low-dose variety on certain children, though larger recruitment will be slowed until “sufficient” safety data on adults has been procured.
Here in the United States, Moderna Inc., Johnson & Johnson, and Novavax are all planning to launch pediatric studies of their COVID-19 vaccines towards the end of 2020.
“If we immunize adolescents – and potentially move down into younger children – we’re going to have the effect of keeping those children from getting infected,” claims Dr. Robert Frank, the director of Cincinnati Children’s Vaccine Research Center.
“But then also they don’t bring the infection home to parents and grandparents.”
It is important to remember that multiple adult trial participants have come down with serious health conditions after getting jabbed for COVID-19. The same can be expected in children who are jabbed, as their immune systems are potentially even more fragile, as they are still developing.
“It is quite important for us to begin the process because this will take some time to do the studies the right way,” claims Dr. Anderson, who is eager to start jabbing children as quickly as possible, even at “warp speed.”
Sources for this article include:
Tagged Under: AstraZeneca, CCP, children, China, China Virus, Chinese Communist Party, Chinese military, Chinese Virus, covid-19, Johnson & Johnson, Moderna, oronavirus, Pfizer, Plandemic, Sinopharm, Sinovac, vaccine, vaccine experiments, vaccines, Wuhan coronavirus
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 VACCINE INJURY NEWS COM